Abstract
Introduction Orelabrutinib (ICP-022) is a novel and highly selective irreversible Bruton's tyrosine kinase (BTK) inhibitor which showed high bioavailability with ~100% BTK occupancy at 24 h at 150 mg daily dosing regimen and demonstrated excellent safety and efficacy profiles in a Phase I/II trial of refractory/relapsed (r/r) chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL). However, the resistant mechanisms of orelabrutinib have not been reported yet.
Methods Deep targeted-gene next generation sequencing (NGS) covering BTK (exon 1-19), PLCG2 (exon 1-33) and high sensitivity droplet digital PCR (ddPCR) detecting BTK Cys481 mutation were assessed in available serial samples in orelabrutinib progression patients in NCT03493217.
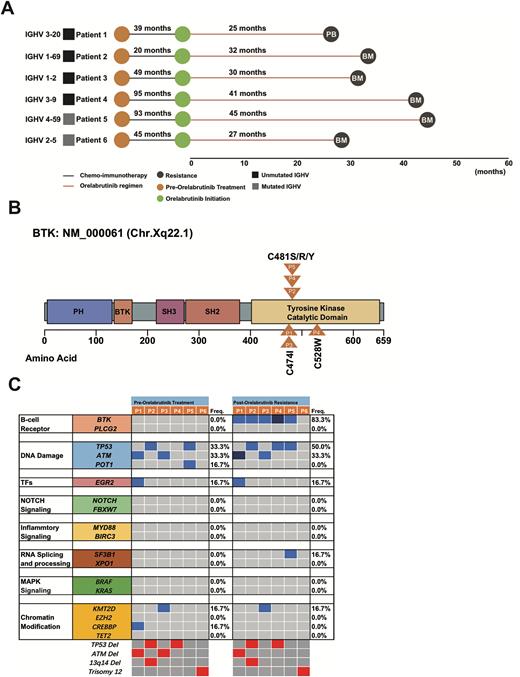
Results We identified 6 patients of our cohort who progressed on orelabrutinib treatment in clinical trial, the median time from orelabrutinib initiation to resistance was 31 months. 3 patients progressed due to lymph nodes enlargement while other 3 patients due to increasing CLL counts in peripheral blood (Figure A). Deep targeted-gene NGS showed that BTK mutations were detected in 5 of 6 patients (5/6, 83.3%, Figure B). Remarkably, BTK kinase domain mutation, BTK Thr474Ile (NM_000061.2: exon15: c.1421C>T: p.T474I) was detected in 2 patients progressed on orelabrutinib. BTK Cys481 mutation was found in other 3 patients progressed on orelabrutinib, among them one patient harboring both BTK Cys481Arg (NM_000061.2: exon15: c.1441T>C: p.C481R) and BTK Leu528Trp (NM_000061.2: exon16: c.1583T>G: p.L528W). In the patient who harbored both BTK C481R and BTK L528W, the variant allele frequency (VAF) of BTK L528W were significantly than BTK L528W (median BTK L528W 9.16% vs. BTK C481S 3.45%). Assessment of the BTK Cys481 mutation with ddPCR confirmed the above results.
Longitudinal NGS analysis of the six patients before orelabrutinib initiation and after orelabrutinib resistances was conducted and the mutational landscape was shown in Figure C. TP53 mutation detected in 2 patients (one of them harbored TP53 Deletion) before orelabrutinib initiation remained existence after orelabrutinib resistance while 1 patient with TP53 deletion before orelabrutinib initiation evolved novel TP53 mutation clone.
Conclusion: We firstly described the BTK mutations occurring in patients with CLL progressing on Orelabrutinib. BTK T474I and BTK L528W were found enriched, suggesting its different mechanisms from the first-generation BTK inhibitor, ibrutinib. Moreover, BTK T474I and L528W were reported to result in resistance to LOXO-305 while ibrutinib could partly overcome the resistance cause by BTK T474I and L528W, indicating different treatment options after orelabrutinib resistance.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal